TenJet
TenJet, also known as percutaneous tenotomy, is a minimally invasive treatment for chronic tendon injuries and calcifications. TenJet’s minimally invasive technology allows physicians to provide patients with a rapid and precise option for removing chronic tendon pain without open surgery or lengthy and tedious treatment plans. The TenJet System bridges the gap in treatment options for tendinosis by enabling an image-guided, tissue-selective resection of degenerative tendon tissue to target the source of chronic tendon pain and provide patients relief from tendinosis, also referred to as chronic tendinitis or chronic tendinopathy.
The procedure is minimally invasive and performed using local anesthesia, requiring only a 1.65mm microincision, which reduces the risk of infection. TenJet’s innovative design utilizes a dual-lumen, 12-gauge needle to deliver a pressurized, high-velocity jet of saline that acts as a cutting water blade to resect and evacuate diseased tendinopathic tissue while leaving the surrounding healthy tissue unharmed.
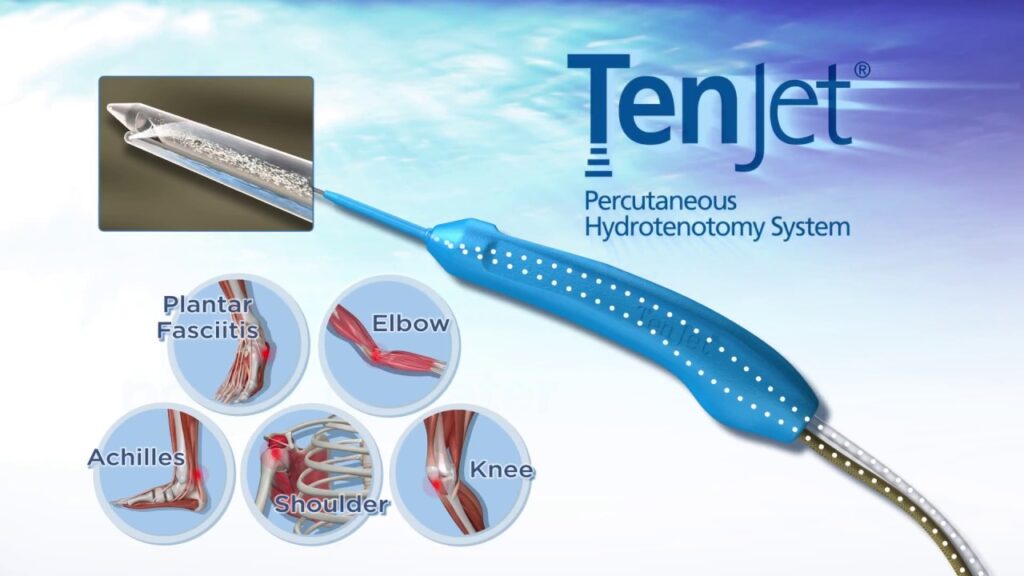
TenJet enables physicians to treat calcifications, tendon thickening, and degeneration associated with tendinosis in an efficient yet precise manner, requiring only an outpatient setting to perform the procedure. During this less than 30-minute procedure, physicians can effectively remove only the diseased tissue and leave healthy tissue behind during real-time, image-guided procedures. Because an ultrasound-guided tenotomy using TenJet requires only a stab incision, the surgical site can be easily closed with Steri-Strips and, in most cases, will not require sutures. Patients can expect minimal downtime and a gradual improvement in pain relief over a 3-month span.
F.A.Q
Interested in finding out more about TenJet and if this treatment could help you?
Give our office a call 631-689-6698 ext 2204.
If you have already explored traditional treatments and have been in pain for over 3 months, you may be ready for a tenotomy by a surgeon who uses TenJet technology. Unlike medical or physical therapies, which primarily only increase blood flow, the goal of TenJet’s minimally invasive technology is to remove the source of pain, the damaged tissue inside your body, but without involving open surgery. This is a relatively safer option than open surgery. The use of TenJet technology for your tenotomy requires only a local anesthetic to numb the area. Typically, you do not need general anesthesia (to be put to sleep) as you would in traditional open surgery. When the procedure is completed, only a small adhesive bandage is used. In most cases, no sutures or stitches are needed.
Typically, a local anesthetic will be used to numb the area, which may cause you to feel a momentary bee sting-type pain before the procedure. However, you should not feel any pain during the procedure, only a slight pressure. During your recovery, you may be sore in the treated area for a short period of time. Over-the-counter pain medication is usually all that is needed for the discomfort.
Unlike traditional open surgeries that may take 6 months or longer for recovery, TenJet’s minimally invasive technology recovery period is expected to be just 4-6 weeks. After consulting with your doctor, you should be able to return to your normal activities. Your healthcare professional will provide complete instructions after the treatment. Please note that your individual results may vary.
If you feel any discomfort after the treatment, you can take an over-the-counter pain medication. After your procedure, your doctor will provide specific instructions based on your condition, work, and lifestyle.
TenJet’s minimally invasive technology is an effective and innovative breakthrough for the treatment of chronic tendon pain, eliminating the need for traditional surgery. The technology was developed in collaboration with the world-renowned Mayo Clinic and was cleared for sale by the Food and Drug Administration (FDA) in the United States in 2013. Furthermore, the technology has been tested in clinical settings with as much as 3 years of positive follow-up on patients.